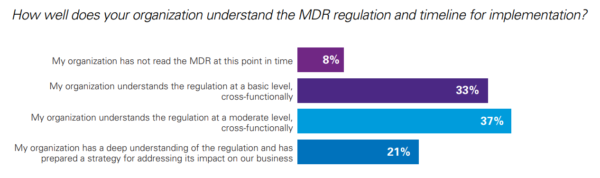
In a survey published by KPMG and the RASP (Regulatory Affairs Professional Society), more than 200 Quality and Regulatory Affairs managers were asked how prepared they feel regarding the coming EU MD Regulation. The companies that took part in the survey range from small structures to international corporations.
- 78% of the companies do not have a sufficient understanding of the regulation,
- 58% have no strategy in place to remediate gaps in their clinical data or post-market data collection,
- 39% have yet to document the roles and responsabilities of the Person Responsible for Regulatory Compliance.
The companies are aware that the late notification of the notified bodies and their restricted number may cause congestion issues in 2020.
The report concludes on a good note: there are 18 months left to plan the compliance actions and to select the products that should be certified in priority.
